Call for Abstract
Scientific Program
Conference Dialysis & Renal Replacement Therapy Trends, will be organized around the theme “Raising Awareness and Promoting Research in Dialysis”
Dialysis Conference 2019 is comprised of 15 tracks and 181 sessions designed to offer comprehensive sessions that address current issues in Dialysis Conference 2019.
Submit your abstract to any of the mentioned tracks. All related abstracts are accepted.
Register now for the conference by choosing an appropriate package suitable to you.
While healthy kidneys have several functions in the body, the most well-known job is to produce urine. When kidney function goes below 10% to 15% kidneys are no longer able to filter the blood and make urine. This causes toxins to build up in the body along with excess fluid. Fortunately, we live in a time when there are treatments and medicines that can replace the functions of the kidneys and keep the body alive. One type of renal replacement therapy — meaning a treatment that replaces kidney function — is hemodialysis. Hemodialysis is a therapy that filters waste, removes extra fluid and balances electrolytes (sodium, potassium, bicarbonate, chloride, calcium, magnesium and phosphate).


How is hemodialysis done?
In hemodialysis, blood is removed from the body and filtered through a man-made membrane called a dialyzer, or artificial kidney, and then the filtered blood is returned to the body. The average person has about 10 to 12 pints of blood; during dialysis only one pint (about two cups) is outside of the body at a time. To perform hemodialysis there needs to be an access created to get the blood from the body to the dialyzer and back to the body. There are three access types for hemodialysis: arteriovenous (AV) fistula, AV graft and central venous catheter. The AV fistula is the vascular access most recommended by the dialysis community; however, you and your doctor will decide which access is best for you.
When a patient goes to hemodialysis, a nurse or technician will check vital signs and get the patient’s weight. The weight gain will tell how much excess fluid the patient has to have removed during the treatment. The patient is then “put on the machine”. Patient with a vascular access (AV fistula or AV graft) will get two needle sticks in their access; one needle takes blood out of the body, the other needle puts it back. Patients with a central venous catheter will have the two tubes from their access connected to the blood tubes that lead to the dialyzer and back to the body. Once the patient is “put on the machine”, the dialysis machine is programmed and then treatment begins.
Blood never actually goes through the dialysis machine. The dialysis machine is like a big computer and a pump. It keeps track of blood flow, blood pressure, how much fluid is removed and other vital information. It mixes the dialysate, or dialysis solution, which is the fluid bath that goes into the dialyzer. This fluid helps pull toxins from the blood, and then the bath goes down the drain. The dialysis machine has a blood pump that keeps the blood flowing by creating a pumping action on the blood tubes that carry the blood from the body to the dialyzer and back to the body. The dialysis machine also has many safety detection features. If you visit a dialysis center, you will likely hear some of the warning sounds made by a dialysis machine.
How does hemodialysis work?
The dialyzer is the key to hemodialysis. The dialyzer is called the artificial kidney because it filters the blood — a job the kidneys used to do. The dialyzer is a hollow plastic tube about a foot long and three inches in diameter that contains many tiny filters. (Dialyzers are made in different sizes so doctors can prescribe the best one for their patients.) There are two sections in the dialyzer; the section for dialysate and the section for the blood. The two sections are divided by a semipermeable membrane so that they don’t mix together. A semipermeable membrane has microscopic holes that allow only some substances to cross the membrane. Because it is semipermeable, the membrane allows water and waste to pass through, but does not allow blood cells to pass through.
Dialysate, also called dialysis fluid, dialysis solution or bath, is a solution of pure water, electrolytes and salts, such as bicarbonate and sodium. The purpose of dialysate is to pull toxins from the blood into the dialysate. The way this works is through a process called diffusion. In the blood of the hemodialysis patient, there is a high concentration of waste, while the dialysate has a low concentration of waste. Due to the difference in concentration, the waste will move through the semipermeable membrane to create an equal amount on both sides. The dialysis solution is then flushed down the drain along with the waste. The electrolytes in the dialysis solution are also used to balance the electrolytes in the patient’s blood. The extra fluid is removed through a process called filtration. The fluid is pushed off by higher pressure on the blood side than on the dialysate side.
How often is hemodialysis done?
Blood needs to flow through the dialyzer for several hours to adequately clean the blood and rid the body of excess fluid. Traditional, in-center hemodialysis is generally done three times a week for about four hours each session. Your doctor will prescribe how long your treatments will be, usually between 3 to 5 hours, but most common is 4 hours. Talk to your doctor about how long you should be on hemodialysis. Some people feel that dialysis lasts a long time; however, healthy kidneys work 24 hours a day, 7 days a week and dialysis must do the job in only 12 or so hours a week.
Alternative hemodialysis schedules include nocturnal and short daily. Normally, these treatments are performed by people who do home hemodialysis. With nocturnal hemodialysis, the patient has dialysis for about eight hours overnight while sleeping. This is a longer, gentler treatment so patients say they have fewer problems with cramping and the “washed out” feeling reported after traditional hemodialysis. More dialysis centers are beginning to offer in-center nocturnal hemodialysis based on reports of patients feeling better about their quality of life and having good lab results. Short daily hemodialysis is performed five or six times per week for about two to three hours each treatment. Talk to your doctor if you are interested in home hemodialysis (HHD) or in-center nocturnal dialysis. You may want to ask your social worker if addition treatments, such as the longer nocturnal hemodialysis and short daily hemodialysis will be covered through your insurance.
Advantages and disadvantages of hemodialysis
Hemodialysis is an effective treatment for those with end stage renal disease. However, hemodialysis alone will not provide a complete treatment for those with kidney failure. Diet and fluid restrictions need to be followed, and medicines may need to be taken to replace other functions of the kidneys, such as regulating blood pressure and stimulating production of red blood cells to prevent anemia.
For those who choose in-center hemodialysis, some of the benefits are that they will have their treatments performed in a dialysis center by trained professionals. They can spend their time in dialysis sleeping, reading, writing, watching television, listening to music or doing other quiet activities. There are four days a week when they will not have to go to dialysis. Some of the disadvantages are that they will have to travel to and from hemodialysis three times each week and it takes advanced notice to travel and arrange for dialysis in a visiting dialysis center. The diet restrictions include limiting foods that contain phosphorus, potassium and sodium and drinking a limited amount of fluid. Some people report a “washed out” feeling after hemodialysis and go home to take a nap. Those who perform nocturnal hemodialysis (in center or at home) report that this washed out feeling is not as common. Also, because nocturnal dialysis is performed during nonproductive sleeping hours, many people report they feel that their lives are more “normal” because they don’t have to take time out of their days for dialysis.
People who choose to perform hemodialysis at home say they enjoy the feeling of control they have over their lives. Instead of going to the dialysis center at a certain time, they can choose when to perform hemodialysis around their schedule.
Risks
Most people who require hemodialysis have a variety of health problems. Hemodialysis prolongs life for many people, but life expectancy for people who need it is still less than that of the general population.
While hemodialysis treatment can be efficient at replacing some of the kidney's lost functions, you may experience some of the related conditions listed below, although not everyone experiences all of these issues. Your dialysis team can help you deal with them.
- Low blood pressure (hypotension). A drop in blood pressure is a common side effect of hemodialysis, particularly if you have diabetes. Low blood pressure may be accompanied by shortness of breath, abdominal cramps, muscle cramps, nausea or vomiting.
- Muscle cramps. Although the cause is not clear, muscle cramps during hemodialysis are common. Sometimes the cramps can be eased by adjusting the hemodialysis prescription. Adjusting fluid and sodium intake between hemodialysis treatments also may help prevent symptoms during treatments.
- Itching. Many people who undergo hemodialysis have itchy skin, which is often worse during or just after the procedure.
- Sleep problems. People receiving hemodialysis often have trouble sleeping, sometimes because of breaks in breathing during sleep (sleep apnea) or because of aching, uncomfortable or restless legs.
- Anemia. Not having enough red blood cells in your blood (anemia) is a common complication of kidney failure and hemodialysis. Failing kidneys reduce production of a hormone called erythropoietin (uh-rith-roe-POI-uh-tin), which stimulates formation of red blood cells. Diet restrictions, poor absorption of iron, frequent blood tests, or removal of iron and vitamins by hemodialysis also can contribute to anemia.
- Bone diseases. If your damaged kidneys are no longer able to process vitamin D, which helps you absorb calcium, your bones may weaken. In addition, overproduction of parathyroid hormone — a common complication of kidney failure — can release calcium from your bones.
- High blood pressure (hypertension). If you consume too much salt or drink too much fluid, your high blood pressure is likely to get worse and lead to heart problems or strokes.
- Fluid overload. Since fluid is removed from your body during hemodialysis, drinking more fluids than recommended between hemodialysis treatments may cause life-threatening complications, such as heart failure or fluid accumulation in your lungs (pulmonary edema).
- Inflammation of the membrane surrounding the heart (pericarditis). Insufficient hemodialysis can lead to inflammation of the membrane surrounding your heart, which can interfere with your heart's ability to pump blood to the rest of your body.
- High potassium levels (hyperkalemia). Potassium is a mineral that is normally removed from your body by your kidneys. If you consume more potassium than recommended, your potassium level may become too high. In severe cases, too much potassium can cause your heart to stop.
- Access site complications. Potentially dangerous complications ― such as infection, narrowing or ballooning of the blood vessel wall (aneurysm), or blockage ― can impact the quality of your hemodialysis. Follow your dialysis team's instructions on how to check for changes in your access site that may indicate a problem.
- Amyloidosis. Dialysis-related amyloidosis develops when proteins in blood are deposited on joints and tendons, causing pain, stiffness and fluid in the joints. The condition is more common in people who have undergone hemodialysis for more than five years.
- Depression. Changes in mood are common in people with kidney failure. If you experience depression or anxiety after starting hemodialysis, talk with your health care team about effective treatment options.
How you prepare
Preparation for hemodialysis starts several weeks to months before your first procedure. To allow for easy access to your bloodstream, a surgeon will create a vascular access. The access provides a mechanism for a small amount of blood to be safely removed from your circulation and then returned to you in order for the hemodialysis process to work. The surgical access needs time to heal before you begin hemodialysis treatments.
There are three types of accesses:
- Arteriovenous (AV) fistula. A surgically created AV fistula is a connection between an artery and a vein, usually in the arm you use less often. This is the preferred type of access because of effectiveness and safety.
- AV graft. If your blood vessels are too small to form an AV fistula, the surgeon may instead create a path between an artery and a vein using a flexible, synthetic tube called a graft.
- Central venous catheter. If you need emergency hemodialysis, a plastic tube (catheter) may be inserted into a large vein in your neck or near your groin. The catheter is temporary.
You can receive hemodialysis in a dialysis center, at home or in a hospital. The frequency of treatment varies, depending on your situation:
- In-center hemodialysis. Many people get hemodialysis three times a week in sessions of three to five hours each.
- Daily hemodialysis. This involves more-frequent, but shorter sessions — usually performed at home six or seven days a week for about two to three hours each time.
Simpler hemodialysis machines have made home hemodialysis less cumbersome, so with special training and someone to help you, you may be able to do hemodialysis at home. You may even be able to do the procedure at night while you sleep.
There are dialysis centers located throughout the United States and in some other countries, so you can travel to many areas and still receive your hemodialysis on schedule. Your dialysis team can help you make appointments at other locations, or you can contact the dialysis center at your destination directly. Plan ahead to make sure space is available and proper arrangements can be made.
The procedure
During treatments, you sit or recline in a chair while your blood flows through the dialyzer ― a filter that acts as an artificial kidney to clean your blood. You can use the time to watch TV or a movie, read, nap, or perhaps talk to your "neighbors" at the center. If you receive hemodialysis at night, you can sleep during the procedure.
- Preparation. Your weight, blood pressure, pulse and temperature are checked. The skin covering your access site — the point where blood leaves and then re-enters your body during treatment — is cleansed.
- Starting. During hemodialysis, two needles are inserted into your arm through the access site and taped in place to remain secure. Each needle is attached to a flexible plastic tube that connects to a dialyzer. Through one tube, the dialyzer filters your blood a few ounces at a time, allowing wastes and extra fluids to pass from your blood into a cleansing fluid called dialysate. The filtered blood returns to your body through the second tube.
- Symptoms. You may experience nausea and abdominal cramps as excess fluid is pulled from your body — especially if you have hemodialysis only three times a week rather than more often. If you're uncomfortable during the procedure, ask your care team about minimizing side effects by such measures as adjusting the speed of your hemodialysis, your medication or your hemodialysis fluids.
- Monitoring. Because blood pressure and heart rate can fluctuate as excess fluid is drawn from your body, your blood pressure and heart rate will be checked several times during each treatment.
- Finishing. When hemodialysis is completed, the needles are removed from your access site and a pressure dressing is applied to prevent bleeding. Your weight may be recorded again. Then you're free to go about your usual activities until your next session.
Results
If you had sudden (acute) kidney injury, you may need hemodialysis only for a short time until your kidneys recover. If you had reduced kidney function before a sudden injury to your kidneys, the chances of full recovery back to independence from hemodialysis are lessened.
Although in-center, three-times-a-week hemodialysis is more common, some research suggests that home dialysis is linked to:
- Better quality of life
- Increased well-being
- Reduced symptoms and less cramping, headaches and shortness of breath
- Improved appetite, sleeping patterns, energy level and ability to concentrate
Your hemodialysis care team monitors your treatment to make sure you're getting the right amount of hemodialysis to remove enough wastes from your blood. Your weight and blood pressure are monitored very closely before, during and after your treatment. About once a month, you'll receive these tests:
- Blood tests to measure urea reduction ratio (URR) and total urea clearance (Kt/V) to see how well your hemodialysis is removing waste from your body
- Blood chemistry evaluation and assessment of blood counts
- Measurements of the flow of blood through your access during hemodialysis
Your care team may adjust your hemodialysis intensity and frequency based, in part, on test results.
Between treatments
Between hemodialysis treatments you can help achieve the best possible results from your hemodialysis by:
- Eating the right foods. Eating properly can improve your hemodialysis results and your overall health. While you're receiving hemodialysis, you'll need to carefully monitor your intake of fluids, protein, sodium, potassium and phosphorus. A dietitian can help you develop an individualized meal plan based on your weight, personal preferences, remaining kidney function and other medical conditions, such as diabetes or high blood pressure.
- Taking your medications as prescribed. Carefully follow the instructions from your health care team.
- Allowing your team to assist you by discussing your concerns. Your health care team can present options to you and help you deal with any concerns.
- Track 1-1Biochemical mechanisms involved in blood-hemodialysis membrane interactions
- Track 1-2Clinical consequences of hemodialysis membrane biocompatibility
- Track 1-3Complications of hemodialysis in the older patient
- Track 1-4Contaminants in water used for hemodialysis
- Track 1-5Hemodialysis in the older adult
- Track 1-6Maintaining water quality for hemodialysis
- Track 1-7Overview of the hemodialysis apparatus
- Track 1-8Plasmapheresis with hemodialysis equipment
- Track 1-9Psychiatric illness in dialysis patients
- Track 1-10Reactions to the hemodialysis membrane
- Track 1-11Reuse of dialyzers
- Track 1-12Serum enzymes in patients with renal failure
- Track 1-13Ultrapure dialysis fluid
- Track 1-14Water purification systems in hemodialysis
Peritoneal dialysis (PD) is a treatment that uses the lining of your abdomen (belly area), called your peritoneum, and a cleaning solution called dialysate to clean your blood. Dialysate absorbs waste and fluid from your blood, using your peritoneum as a filter. One benefit of PD is that it is not done in a dialysis center. You can do your PD treatment any place that is clean and dry. This can allow you more freedom to work, travel or do other activities you enjoy without worrying about scheduling dialysis appointments. The two most common types of PD are continuous ambulatory PD (CAPD) and continuous cycler-assisted PD (CCPD). Your doctor can help you decide which is right for you.


The benefits of peritoneal dialysis compared with hemodialysis can include:
- Greater lifestyle flexibility and independence. These can be especially important if you work, travel or live far from a hemodialysis center.
- More flexible dietary guidelines. Peritoneal dialysis is done more continuously than hemodialysis, resulting in less accumulation of potassium, sodium and fluid.
- More stable blood chemistry and body hydration. Peritoneal dialysis doesn't require intravenous (IV) access, which can disrupt your circulation and fluid levels.
- Longer lasting residual kidney function. People who use peritoneal dialysis might retain kidney function slightly longer than people who use hemodialysis.
Risks
Complications of peritoneal dialysis can include:
- Infections. An infection of the abdominal lining (peritonitis) is a common complication of peritoneal dialysis. An infection can also develop at the site where the catheter is inserted to carry the cleansing fluid (dialysate) into and out of your abdomen. The risk of infection is greater if the person doing the dialysis isn't adequately trained.
- Weight gain. The dialysate contains sugar (dextrose). Absorbing some of the dialysate might cause you to take in several hundred extra calories a day, leading to weight gain. The extra calories can also cause high blood sugar, especially if you have diabetes.
- Hernia. Holding fluid in your abdomen for long periods may strain your muscles.
- Inadequate dialysis. Peritoneal dialysis can become ineffective after several years. You might need to switch to hemodialysis.
If you have peritoneal dialysis, you'll need to avoid:
- Certain prescription and over-the-counter medications that can damage your kidneys, including nonsteroidal anti-inflammatory drugs.
- Soaking in a bath or hot tub, or swimming in a lake, pond, river or nonchlorinated pool — which increases the risk of infection. Showers and swimming in a chlorinated pool are generally acceptable.
How you prepare
You'll receive training on what peritoneal dialysis involves and how to use the equipment.
You'll also need an operation to insert the catheter that carries the dialysate in and out of your abdomen. The insertion might be done under local or general anesthesia. The tube is usually inserted near your bellybutton.
After the tube is inserted, your doctor will probably recommend waiting at least two weeks before starting peritoneal dialysis treatments to give the catheter site time to heal. Complete healing of the catheter site can take up to two months.
What you can expect
During peritoneal dialysis:
- The dialysate flows into your abdomen and stays there for a prescribed period of time (dwell time) — usually four to six hours
- Dextrose in the dialysate helps filter waste, chemicals and extra fluid in your blood from tiny blood vessels (capillaries) in the lining of your abdominal cavity (peritoneum)
- When the dwell time is over, the solution — along with waste products drawn from your blood — drains into a sterile collection bag
The process of filling and then draining your abdomen is called an exchange. Different methods of peritoneal dialysis have different schedules of exchange. The two main schedules are:
- Continuous ambulatory peritoneal dialysis (CAPD)
- Continuous cycling peritoneal dialysis (CCPD)
Continuous ambulatory peritoneal dialysis (CAPD)
You fill your abdomen with dialysate, let it remain there for a prescribed dwell time, then drain the fluid. Gravity moves the fluid through the catheter and into and out of your abdomen.
With CAPD:
- You may need three to five exchanges during the day and one with a longer dwell time while you sleep
- You can do the exchanges at home, work or any clean place
- You're free to go about your normal activities while the dialysate dwells in your abdomen
Continuous cycling peritoneal dialysis (CCPD)
Also known as automated peritoneal dialysis (APD), this method uses a machine (automated cycler) that performs multiple exchanges at night while you sleep. The cycler automatically fills your abdomen with dialysate, allows it to dwell there and then drains it to a sterile bag that you empty in the morning.
With CCPD:
- You must remain attached to the machine for 10 to 12 hours at night.
- You aren't connected to the machine during the day. But in the morning you begin one exchange with a dwell time that lasts the entire day.
- You might have a lower risk of peritonitis because you connect and disconnect to the dialysis equipment less frequently than you do with CAPD.
To determine the method of exchange that's best for you, your doctor will consider your medical condition, lifestyle and personal preferences. Your doctor might suggest certain modifications to individualize your program.
Results
Many factors affect how well peritoneal dialysis works in removing wastes and extra fluid from your blood. These factors include:
- Your size
- How quickly your peritoneum filters waste
- How much dialysis solution you use
- The number of daily exchanges
- Length of dwell times
- The concentration of sugar in the dialysis solution
To check if your dialysis is removing enough waste products, your doctor is likely to recommend:
- Peritoneal equilibration test (PET). This test compares samples of your blood and your dialysis solution during an exchange. The results indicate whether waste toxins pass quickly or slowly from your blood into the dialysate. That information helps determine whether your dialysis would be improved if the solution stayed in your abdomen for a shorter or longer time.
- Clearance test. A blood sample and a sample of used dialysis solution are analyzed to determine how much of a certain waste product (urea) is being removed from your blood during dialysis. If you still produce urine, your doctor may also take a urine sample to measure its urea concentration.
If the test results show that your dialysis schedule is not removing enough wastes, your doctor might change your dialysis routine to:
- Increase the number of exchanges
- Increase the amount of dialysate you use for each exchange
- Use a dialysate with a higher concentration of dextrose
You can improve your dialysis results and your overall health by eating the right foods, including foods low in sodium and phosphorus. A dietitian can help you develop an individualized meal plan based on your weight; your personal preferences; and your remaining kidney function and other medical conditions, such as diabetes or high blood pressure.
Taking your medications as prescribed also is important for getting the best possible results. While you're receiving peritoneal dialysis, you'll likely need various medications to control your blood pressure, stimulate production of red blood cells, control the levels of certain nutrients in your blood and prevent the buildup of phosphorus in your blood.
- Track 2-1Peritoneal dialysis solutions
- Track 2-2Use of peritoneal dialysis for the treatment of acute kidney injury in adults
- Track 2-3Urgent-start peritoneal dialysis
- Track 2-4Tunnel and peritoneal catheter exit site infections in continuous peritoneal dialysis
- Track 2-5Rapid transporters on maintenance peritoneal dialysis
- Track 2-6Problems with solute clearance and ultrafiltration in continuous peritoneal dialysis
- Track 2-7Prescribing and assessing adequate peritoneal dialysis
- Track 2-8Placement and maintenance of the peritoneal dialysis catheter
- Track 2-9Peritoneal equilibration test
- Track 2-10Abdominal hernias in continuous peritoneal dialysis
- Track 2-11Pathophysiology and prevention of peritonitis in peritoneal dialysis
- Track 2-12Noninfectious complications of peritoneal dialysis catheters
- Track 2-13Noninfectious complications of continuous peritoneal dialysis
- Track 2-14Modalities for the diagnosis of abdominal and thoracic cavity defects in peritoneal dialysis patients
- Track 2-15Microbiology and therapy of peritonitis in continuous peritoneal dialysis
- Track 2-16Mechanisms of solute clearance and ultrafiltration in peritoneal dialysis
- Track 2-17Evaluation of hypervolemia in peritoneal dialysis patients
- Track 2-18Bloody peritoneal dialysate (hemoperitoneum)
A vascular access procedure inserts a flexible, sterile plastic tube called a catheter into a blood vessel to allow blood to be drawn from or medication to be delivered to a patient’s bloodstream over an extended period.
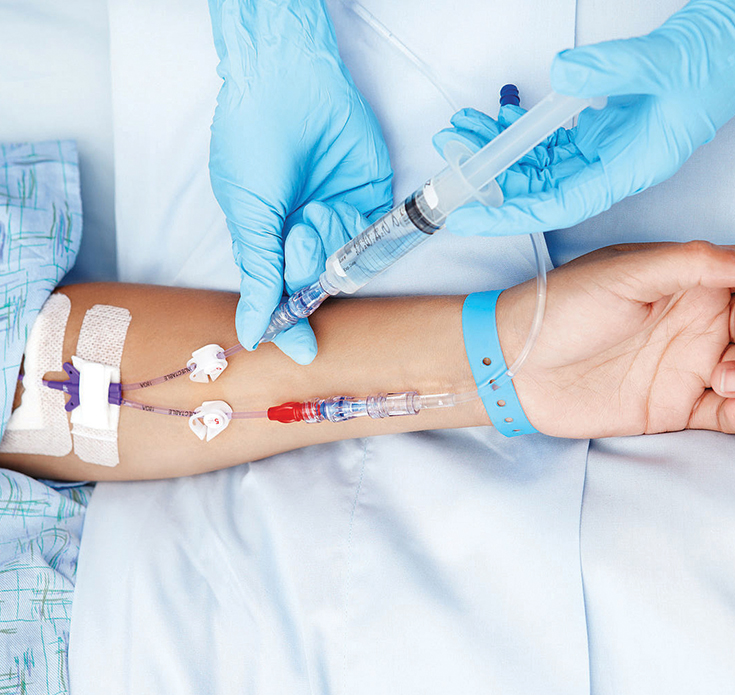
There are three access types for hemodialysis: arteriovenous (AV) fistula, AV graft and central venous catheter. Each access is created surgically. There are a limited number of places on the body where an access can be placed—the arms, legs, neck or chest. The fistula and graft are considered to be permanent accesses because they are placed under the skin with a plan to use them for many years.
- the Arterio Venous (AV) fistula
- the Arterio Venous (AV) graft
- the central venous catheter or internal port devices (LifeSite®)
Each access is created surgically. There are a limited number of places on the body where an access can be placed—the arms, legs, neck or chest.
The fistula and graft are considered to be permanent accesses because they are placed under the skin with a plan to use them for many years. When patients find out they are in the advanced stages of chronic kidney disease and will be starting dialysis in the future, their nephrologist will advise them to get a fistula or graft. Having the access in place well before beginning dialysis will give this lifeline time to "mature," so it can be ready to use.
When patients suddenly discover they have kidney failure, a catheter may be placed to allow for immediate dialysis treatment. The catheter will be used until a fistula or graft has time to mature. A catheter can also be used on a permanent basis, if the patient is unable to have a fistula or graft—but a catheter is always a last resort.
AV Fistula
An AV fistula is created by directly connecting a person’s artery and vein—usually in the arm. This procedure may be performed as an outpatient operation using a local anesthetic. As blood flows to the vein from the newly connected artery, the vein grows bigger and stronger. The patient is taught to do exercises—such as squeezing a rubber ball—to help the fistula strengthen and mature to get it ready for use. This takes anywhere from six weeks to four months or more. Once the fistula has matured, it can provide good blood flow for many years of hemodialysis.
Kidney and hemodialysis experts, including the National Kidney Foundation (NKF), Centers for Medicare and Medicaid Services (CMS), the American Association of Kidney Patients (AAKD) and others consider the fistula the "gold standard" access choice. Research studies have proven patients with a fistula have the fewest complications, such as infection or clotting, compared to all other access choices.
Currently, an access improvement initiative known as "Fistula First" is being sponsored by CMS throughout the United States to support an increase in the use of fistulas for hemodialysis patients.
The fistula is considered the "gold standard" access because it:
- has a lower risk of infections than other access types
- has a lower risk of forming clots than other access types
- performs better than other accesses
- allows for greater blood flow
- lasts longer than the other access types
- can last many years, even decades, when well-cared for
Some issues people may have with fistulas include:
- the appearance of bulging veins at the access site
- taking several months for a new one to mature
- not maturing at all in some cases
Not everyone may be able to have a fistula due to weak arteries, veins or other medical conditions; it is best to discuss your access options with your doctor, but ask for a fistula first.
AV Graft
The AV graft is similar to a fistula, in that it is also an under the skin connection of an artery and vein, except that with a graft, a man-made tubing connects the artery and vein. The soft, plastic-like tube is about one-half inch in diameter and is made from a type of Teflon® or Gore-Tex® material. Transplanted animal or human vessels may also be used as grafts to connect an artery and vein. Grafts are usually placed in the arm, but can also be placed in the thigh.
Grafts do not require as much time to mature as fistulas, because the graft does not need time to enlarge before using. In most cases a graft can be used about two to six weeks after placement. Because grafts are created from materials outside of the body, they tend to have more problems than fistulas due to clotting and infections. Grafts may not last as long as a fistula and could need to be repaired or replaced each year.
Caring for a fistula or graft
Taking good care of your fistula or graft will help keep it working properly. There are a few things you can do to help prevent infections, clotting and damage to your access.
Cleanliness is important to keep out infections
Keep your access area clean and free of any trauma. Look for signs of infection including: pain, tenderness, swelling or redness around your access area. Also, be aware of any fever and flu-like symptoms. If you do get an infection and catch it early, it can usually be treated with antibiotics.
Your dialysis care team will teach you how to carefully wash your access arm before each dialysis treatment. Make sure to wash thoroughly and be sure the care team member specially prepares your access site to prevent infection.
Unrestricted blood flow helps lower the risk of clotting
Protect your access from any restriction or trauma by:
- avoiding tight clothes, jewelry or anything that may put pressure on your access
- not sleeping on top of or resting on your access area
- refraining from carrying purses, bags or heavy items across your access area
- always requesting that blood be drawn from your non-access arm
- always requesting that blood pressure be taken from your non-access arm
Learn the feel of the "thrill" or vibration of blood going through your access and check it several times a day. Call your dialysis care team immediately if the flow stops or changes. This could mean a blood clot. With quick action, many clots can be dissolved or removed.
Learn to listen with a stethoscope to the sound (called "bruit") of blood flowing ("whooshing") through your access. If the sound of the bruit changes to a higher pitch, like a whistle, it could be an indication that blood vessels are narrowing (call stenosis), which may slow or stop blood flow through your access. If you do not hear the bruit at all, or only your pulse, you may have a blood clot in your access. Call your dialysis care team if you notice any change in your access.
Good needle sticks (cannulation) can help keep your access working well
To prevent tearing or damage to your access, it’s recommended that you pay attention to the needle stick locations when you’re being put on dialysis. The arterial and venous needle tips should be at least two inches apart from each other, as well as away from access surgical scars. The new needle stick sites should be at least one-fourth inch from the sites used the time before. Allow about two weeks for healing of previous sites to help maintain the health of the access.
Many people are nervous about having needles placed; however, there are numbing creams that can be used to reduce the pain and fear of needle sticks. Talk to your nephrologist and dialysis care team about ways to decrease pain and to calm anxiety.
Have you ever considered learning how to stick your own access (called self cannulation)? Many patients find they prefer having control of the needle stick process. When you self cannulate, you can control and participate in this part of your vascular access care and treatment. If you’d like to learn how to self-cannulate, ask a dialysis team member. You’ll receive education and training.
After dialysis treatment your needles will be removed and you will need to apply pressure with sterile gauze over your needle sites to stop the bleeding. Your dialysis team will provide you with clean gloves and teach you the proper procedures to stop bleeding as well as prevent infection.
Catheters and Internal Port Devices (LifeSite®)
A catheter is a narrow tube that is placed into a large central vein, usually in the patient’s neck, chest or groin. Placement of the catheter usually takes less than a half hour. Usually, two tubes extend out of the body from the catheter: one allows blood out of the body (arterial port) and one allows blood back into the body (venous port).
Internal port devices are special access systems, which are placed under the skin and connected to very large venous catheters to provide access to remove blood out of the body for cleaning and then back into the body.
Catheters and internal port devices can be used for dialysis immediately after placement. A catheter or internal port device may be used when one must begin dialysis before a fistula or graft has time to mature.
Some patients use permanent catheters, however, kidney and hemodialysis experts, including the National Kidney Foundation (NKF), Centers for Medicare and Medicaid Services (CMS), the American Association of Kidney Patients (AAKD) and others do not recommend catheters and internal port devices for long-term hemodialysis. Concerns with catheters or internal port devices include:
- a greater likelihood to become infected, clotted or fail
- a slower blood flow so dialysis may not clean the blood as thoroughly as with a fistula or graft
- inferior access for long-term hemodialysis
Caring for a catheter and Internal Port Devices (LifeSite®)
Catheters and internal port devices will require care to keep them protected, free of infections and working well.
Cleanliness helps prevent infections
It is very important to always keep your catheter exit site clean and dry. This may mean you cannot swim, take showers or soaking baths. You will need to carefully wash without getting your unhealed catheter exit site wet. Your physician may allow you to shower once your catheter exit site is well healed.
Your dialysis care team will teach you how to protect your catheter or internal port device when it is not being used for dialysis. You will be taught the importance of making sure your catheter clamps are clamped and end-caps are on securely when you’re not dialyzing. These will help decrease your risk of infection and avoid air from getting into your catheter. You will also be taught to check regularly for signs of infection such as redness, swelling, pain, pus or fever. Call your dialysis care team right away if you think you may have an infection.
Special precautions such as wearing a nose and mouth covering masks whenever your catheter is being accessed will help prevent any microbes from your nose or mouth from contaminating the catheter or exit site. Your dialysis care team members will also wash their hands and wear clean gloves when caring for your access.
Protect your catheter
Because your catheter exits and extends from your body, you’ll need to be careful not to pull on or tug it, or the protective dressing. Take care to be gentle around your catheter when getting dressed and undressed or removing a blanket or covering. Always keep sharp instruments, such as scissors, away from your catheter.
- Track 3-1Maturation and evaluation of the newly created hemodialysis arteriovenous fistula
- Track 3-2Thrombosis associated with chronic hemodialysis vascular catheters
- Track 3-3Techniques for angioplasty of the arteriovenous hemodialysis access
- Track 3-4Secondary hemodialysis arteriovenous fistula
- Track 3-5Primary failure of the hemodialysis arteriovenous fistula
- Track 3-6Physical examination of the arteriovenous graft
- Track 3-7Patient evaluation and vascular mapping prior to placement of hemodialysis arteriovenous access
- Track 3-8Overview of hemodialysis arteriovenous graft maintenance and thrombosis prevention
- Track 3-9Overview of hemodialysis arteriovenous fistula maintenance and thrombosis prevention
- Track 3-10Overview of chronic hemodialysis vascular access
- Track 3-11Nonthrombotic complications of arteriovenous hemodialysis access
- Track 3-12Monitoring and surveillance of hemodialysis arteriovenous grafts to prevent thrombosis
- Track 3-13Arteriovenous fistula recirculation in hemodialysis
- Track 3-14Hemodialysis arteriovenous graft dysfunction and failure
- Track 3-15Hemodialysis anticoagulation
- Track 3-16Heart failure and hemodialysis arteriovenous fistulae
- Track 3-17Failure of the mature hemodialysis arteriovenous fistula
- Track 3-18Examination of the mature hemodialysis arteriovenous fistula
- Track 3-19Endovascular intervention for the treatment of stenosis in the arteriovenous access
- Track 3-20Creating an arteriovenous fistula for hemodialysis
- Track 3-21Clinical monitoring and surveillance of the mature hemodialysis arteriovenous fistula
- Track 3-22Central vein stenosis associated with hemodialysis access
- Track 3-23Arteriovenous graft creation for hemodialysis and its complications
When kidneys fail, dialysis is necessary to remove waste products such as urea from the blood. By itself, urea is only mildly toxic, but a high urea level means that the levels of many other waste products that are more harmful and not as easily measured are also building up.
To see whether dialysis is removing enough urea, the dialysis clinic should periodically—normally once a month—test a patient's blood to measure dialysis adequacy. Blood is sampled at the start of dialysis and at the end. The levels of urea in the two blood samples are then compared. Two methods are generally used to assess dialysis adequacy, URR and Kt/V.
What is the URR?
URR stands for urea reduction ratio, meaning the reduction in urea as a result of dialysis. The URR is one measure of how effectively a dialysis treatment removed waste products from the body and is commonly expressed as a percentage.
Example: If the initial, or predialysis, urea level was 50 milligrams per deciliter (mg/dL) and the postdialysis urea level was 15 mg/dL, the amount of urea removed was 35 mg/dL.
50 mg/dL - 15 mg/dL = 35 mg/dL
The amount of urea removed (35 mg/dL) is expressed as a percentage of the predialysis urea level (50 mg/dL).
35/50 = 70/100 = 70%
Although no fixed percentage can be said to represent an adequate dialysis, patients generally live longer and have fewer hospitalizations if the URR is at least 60 percent. As a result, some experts recommend a minimum URR of 65 percent.
The URR is usually measured only once every 12 to 14 treatments, which is once a month. The URR may vary considerably from treatment to treatment. Therefore, a single value below 65 percent should not be of great concern, but a patient's average URR should exceed 65 percent.
What is the Kt/V?
Kt/V is another way of measuring dialysis adequacy. In this measurement,
- K stands for the dialyzer clearance, the rate at which blood passes through the dialyzer, expressed in milliliters per minute (mL/min)
- t stands for time
- Kt, the top part of the fraction, is clearance multiplied by time, representing the volume of fluid completely cleared of urea during a single treatment
- V, the bottom part of the fraction, is the volume of water a patient's body contains
Example: If the dialyzer's clearance is 300 mL/min and a dialysis session lasts for 180 minutes (3 hours), Kt will be 300 mL/min multiplied by 180 minutes. The result comes to 54,000 mL, or 54 liters.
Kt = 300 mL/min multiplied by 180 minutes
Kt = 54,000 mL = 54 liters
The body is about 60 percent water by weight. If a patient weighs 70 kilograms (kg), or 154 pounds (lbs), V will be 42 liters.
V = 70 kg multiplied by .60 = 42 liters
So the ratio—K multiplied by t to V, or Kt/V—compares the amount of fluid that passes through the dialyzer with the amount of fluid in the patient's body. The Kt/V for this patient would be 1.3.
Kt/V = 54/42 = 1.3
How Does the Kt/V Compare with the URR?
The Kt/V is mathematically related to the URR and is in fact derived from it, except that the Kt/V also takes into account two additional factors:
- urea generated by the body during dialysis
- extra urea removed during dialysis along with excess fluid
The Kt/V is more accurate than the URR in measuring how much urea is removed during dialysis, primarily because the Kt/V also considers the amount of urea removed with excess fluid. Consider two patients with the same URR and the same postdialysis weight, one with a weight loss of 1 kg—about 2.2 lbs—during the treatment and the other with a weight loss of 3 kg-about 6.6 lbs. The patient who loses 3 kg will have a higher Kt/V, even though both have the same URR.
The fact that a patient who loses more weight during dialysis will have a higher Kt/V does not mean it is better to gain more water weight between dialysis sessions so more fluid has to be removed, because the extra fluid puts a strain on the heart and circulation. However, patients who lose more weight during dialysis will have a higher Kt/V for the same level of URR.
Peritoneal Dialysis Adequacy
When kidneys fail, waste products such as urea and creatinine build up in the blood. One way to remove these wastes is a process called peritoneal dialysis (PD). The walls of the abdominal cavity are lined with a membrane called the peritoneum. During PD, a mixture of dextrose (sugar), salt, and other minerals dissolved in water, called dialysis solution, is placed in a person's abdominal cavity through a catheter. The body's peritoneal membrane enclosing the digestive organs allows waste products and extra body fluid to pass from the blood into the dialysis solution. These wastes then leave the body when the used solution is drained from the abdomen. Each cycle of draining and refilling is called an exchange. The time the solution remains in the abdomen between exchanges is called the dwell time. During this dwell time, some of the dextrose in the solution crosses the membrane and is absorbed by the body.
Many factors affect how much waste and extra fluid are removed from the blood. Some factors—such as the patient's size and the permeability, or speed of diffusion, of the peritoneum—cannot be controlled. Dialysis solution comes in 1.5-, 2-, 2.5-, or 3-liter bags for manual exchanges and 5- or 6-liter bags for automated exchanges. The dialysis dose can be increased by using a larger fill volume, but only within the limits of the person's abdominal capacity. Everyone's peritoneum filters wastes at a different rate. In some people, the peritoneum does not allow wastes to enter the dialysis solution efficiently enough to make PD feasible.
Other factors that determine how efficiently a person's blood is filtered can be controlled. Controllable factors include the number of daily exchanges and the dwell times. When fresh solution is first placed in the abdomen, it draws in wastes rapidly. As wastes fill the solution, it cleans the blood less efficiently. For example, a patient may perform one exchange with a 6-hour dwell time, during which the solution pulls in nearly as much urea as it can hold. But in the second half of that dwell time, urea is being removed from the blood very slowly. If the patient performed two exchanges with 3-hour dwell times instead, the amount of urea removed would be substantially greater than that removed in one 6-hour dwell time.
Another way to increase the amount of fluid and waste drawn into the peritoneal cavity is to use dialysis solution with a higher concentration of dextrose. Dialysis solution comes in 1.5 percent, 2.5 percent, and 4.25 percent dextrose concentrations. A higher dextrose concentration moves fluid and more wastes into the abdominal cavity, increasing both early and long-dwell exchange efficiency. Eventually, however, the body absorbs dextrose from the solution. As the concentration of dextrose in the body comes closer to that in the solution, dialysis becomes less effective, and fluid is slowly absorbed from the abdominal cavity.
Types of Peritoneal Dialysis
The two types of peritoneal dialysis differ mainly in the schedule of exchanges. In continuous ambulatory peritoneal dialysis (CAPD), the patient empties a fresh bag of dialysis solution into the abdomen. After 4 to 6 hours of dwell time, the patient returns the solution containing wastes to the bag. The patient then repeats the cycle with a fresh bag of solution. CAPD does not require a machine; the process uses gravity to fill and empty the abdomen. A typical prescription for CAPD requires three or four exchanges during the day and one long—usually 8 to 10 hours—overnight dwell time as the patient sleeps. The dialysis solution used for the overnight dwell time may have a higher concentration of dextrose so that it removes wastes and fluid for a longer time.
Continuous ambulatory peritoneal dialysis (CAPD) is the most common form of peritoneal dialysis.
To remove even more wastes, a mini-cycler machine can be used to exchange the dialysis solution once or several times overnight as the patient sleeps. Such additional exchanges may also help prevent the body from absorbing excessive amounts of dextrose and dialysis solution from the overnight dwell time.
Continuous cycler-assisted peritoneal dialysis (CCPD) uses a machine to fill and empty the abdomen three to five times during the night while the person sleeps. In the morning, the last fill remains in the abdomen with a dwell time that lasts the entire day. Sometimes one additional exchange is done in the mid-afternoon to increase the amount of waste removed and to prevent excessive absorption of fluid. The dialysis solution used for the long daytime dwell may have a higher concentration of dextrose.
Testing for Efficiency
The tests to see whether the exchanges are removing enough urea are especially important during the first weeks of dialysis, when the health care team needs to determine whether the patient is receiving an adequate amount, or dose, of dialysis.
The peritoneal equilibration test—often called the PET—measures how much dextrose has been absorbed from a bag of infused dialysis solution and how much urea and creatinine have entered into the solution during a 4-hour dwell. The peritoneal transport rate varies from person to person. People who have a high rate of transport absorb dextrose from the dialysis solution quickly, and they should be given a dialysis schedule that avoids exchanges with a long dwell time because they tend to absorb too much dextrose and dialysis solution from such exchanges.
In the clearance test, samples of used solution drained over a 24-hour period are collected, and a blood sample is obtained during the day when the solution is collected. The amount of urea in the solution is compared with the amount in the blood to see how effective the current PD schedule is in clearing the blood of urea. If the patient has more than a few ounces of urine output per day, the urine should also be collected during this period to measure its urea concentration.
From the used solution, urine, and blood measurements, one can compute a urea clearance, called Kt/V, and a creatinine clearance rate—normalized to body surface area. The residual clearance of the kidneys is also considered. Based on these measurements, one can determine whether the PD dose is adequate.
If the laboratory results show that the dialysis schedule is not removing enough urea and creatinine, the doctor may change the prescription by
- increasing the number of exchanges per day for patients treated with CAPD or per night for patients treated with CCPD
- increasing the volume—amount of solution in the bag—of each exchange in CAPD
- adding an extra, automated middle-of-the-night exchange to the CAPD schedule
- adding an extra middle-of-the-day exchange to the CCPD schedule
- using a dialysis solution with a higher dextrose concentration
Compliance
One of the big problems with PD is that patients sometimes do not perform all of the exchanges recommended by their medical team. They either skip exchanges or sometimes skip entire treatment days when using CCPD. Skipping PD treatments has been shown to increase the risk of hospitalization and death.
Residual Kidney Function
Normally the PD prescription factors in the amount of residual kidney function. Residual function typically falls, although slowly, over the months or even years of treatment with PD. This means that, more often than not, the number of PD exchanges prescribed, or the volume of exchanges, needs to be increased as residual function falls.
The doctor should determine the patient's dose of PD on the basis of practice guidelines published by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (K/DOQI) (see For More Information). Health care providers should work closely with their patients to ensure that the proper PD dose is administered. To maximize health and prolong life, patients should follow instructions carefully to get the most out of their dialysis exchanges.
- Track 4-1Incorporating residual kidney function into the dosing of intermittent hemodialysis
- Track 4-2Patient survival and maintenance dialysis
- Track 4-3Prescribed versus delivered dialysis: Importance of dialysis time
- Track 4-4Prescribing and assessing adequate hemodialysis
- Track 4-5Prescribing and assessing adequate peritoneal dialysis
- Track 4-6Protein intake in maintenance hemodialysis patients
- Track 4-7Rapid transporters on maintenance peritoneal dialysis
- Track 4-8Urine output and residual kidney function in kidney failure
Once the kidney fails, there are four options. None of these options are a cure but a form of treatment. The four types of treatment will be discussed below. Kidney transplant, peritoneal dialysis (PD), hemodialysis (HD), and palliative care.
Kidney Transplant
Surgery that involves transplanting one healthy kidney from a living donor or a deceased donor. Transplant is not a cure for kidney failure but another form of treatment. As a candidate for kidney transplant a rigorous pre-transplant evaluation is done by a transplant center to determine your candidacy. There are three different ways to receive a kidney transplant 1. Living donor from family/friend/stranger; 2. Donor exchange program; 3. Deceased donor (free kidney). To receive a kidney from a deceased donor, wait time depends on the state. In California, the wait time for a deceased donor is approximately 5-7 years. Talk to your transplant center to have a better understanding of the wait time. The advantages of having a kidney transplant are no longer having to do dialysis, normal diet without out having to take phosphorus binders with each meal, regularly feeling normal/increased energy, most important a better quality of life. After having a kidney transplant, anti-rejection medications will be required for the life of the transplanted kidney to prevent the body from attacking the organ causing rejection.
Peritoneal Dialysis (PD)
This is a type of dialysis which the patient’s peritoneum is used as the dialysis membrane. The peritoneum is an anatomical structure which covers the abdominal organs. This natural organ filters the toxins and excretes waste from the body. The peritoneum has two layers which together forms a “pocket” called peritoneal cavity. Fluid is instilled into the peritoneal cavity using peritoneal catheter (Peritoneal catheter is implanted by the surgeon into the abdominal wall.). The dialysis fluid is instilled into the cavity by either gravity (CAPD) or a machine called cycler (CCPD). The fluid is left in for several hours, during that time, waste materials and excess fluids pass through the membrane and are then drained from the cavity via the peritoneal catheter. No needles are involved for this type of dialysis. It is done primarily during the night when you are sleeping. Once medical advantage is that is preserves your veins and arteries for future use. Patient of this type of modality have better blood chemistries, flexible schedules, independence, and above all are able to travel to anywhere in the world. It is also the self-esteem due to empowerment of the patient. More liberal diet compared to other methods, better “feel-good feelings” compared to hemodialysis and research has shown that patient of this modality has better transplant outcome compared to hemodialysis. The downside of this modality is that it requires home supplies and storage space, risk for infection, and requires dialyzing daily.
Hemodialysis
Is a medical treatment in which an artificial filter outside the body is used to clean the blood. An access is needed to remove blood from the body to the machine which is attached to an artificial filter. Two needles are need for hemodialysis. One is to remove the blood through the access to the machine for filtering. Another needle is needed to return the filter blood back to the body. The blood is pumped passed a semipermeable artificial dialysis membrane called a dialyzer. Poisons and toxins removed through this method are diffused into a liquid called dialysate. The dialysate are then discarded along with the toxin; the purified blood is then return to the body. Because the patient’s blood is outside the body, for this process, the dialysis machine has a warmer to keep the blood at body temperature. Once the blood is outside the body in the tubing system, there is a tendency to form clots which can be fatal. A blood thinner called heparin is given to prevent the clotting in the plastic tubing. Hemodialysis can be done in three ways:
- In-Center hemodialysis
- Home hemodialysis
- Nocturnal dialysis
In-center hemodialysis is usually done at a dialysis facility usually three to four times per week. These sessions last three to four hours and are done during the day. Nurses and technicians are involved in setting up and operating the dialysis machine. The main advantage of in-center hemodialysis are patients are able to meet nurses three times per week and emergency are address immediately, social support from in-center, and patients are not directly involved in their care. The disadvantage of in-center hemodialysis are the treatment schedules are strict, lack of independence, are at high risk for nosocomial infection due to exposures to other patients, difficult traveling. Overall, hemodialysis as a modality has several disadvantages.
Nocturnal hemodialysis is done at night in-center, sessions usually last six to eight hours during the night. Nurses and technicians operate the dialysis machine. The advantages and disadvantages for this modality are the same as in-center.
Home hemodialysis is done about five to six times per week. Sessions are 2.5-3hrs during the day; a partner is required for this modality. The partner could be a spouse, a family member, a caregiver, or a paid hemodialysis technicians or a nurse. Training usually takes about one month for this method. The advantages of home hemodialysis are being include independence and convenience of dialyzing at home.. It has a relatively flexible schedule compared to in-center hemodialysis. Traveling is relatively possible compared to in-center hemodialysis. The disadvantages for this modality are: treatments are done during the day, frequent needle sticks, and the need for having a partner.
The main disadvantage for hemodialysis is a permanent access such as a fistula or graft is required. There are greater risk for clots and infections. It can also cause steal syndrome. The fistula can decrease the amount of blood flow to the hand, making it painful, cold, and pale. It is called steal syndrome because the fistula steals blood from the distal part of the hand. Doctor’s refer to this as ischemia. Fistula and grafts remains permanent in the body and are difficult to remove. Frequent clotting can lead to exhaustion of vascular access for future use.
The term used in the dialysis world to describe the various available treatment options is “modality”...but—especially in dialysis—“modality” is an increasingly dirty word. Not dirty as in smutty, but dirty as in confused, confusing, and unclear.
So, what does modality really mean in dialysis 2017? Vanishingly less, I fear, as the margins between options—and within options—become increasingly blurred.
Once upon a time, the term “treatment modality” described one of four relatively clear and well-demarcated choices:
- Peritoneal Dialysis (PD)
- Haemodialysis (HD)
- Transplantation (Tx)
- Conservative Care (CC)
But, then it started to get complicated. Indeed, by 2007, I felt it had become so complicated that I asked Mark Macgregor (UK) and Chris Blagg (USA) to co-author a paper(1) that focused on the developing difficulties (back then) with the burgeoning terminology of dialysis. Little did we know then that it would get worse, and not better, in the decade that followed! Here are just some of the changes since 2007...
First, Peritoneal Dialysis (PD)
I am purposefully glossing over the very early days when PD was done as an inpatient in hospital (commonly 3 days a week by intermittent “stick” catheter insertion) and was called intermittent PD (IPD). Though, it is important to acknowledge that early time of IPD if we are to understand why the term “continuous” was later introduced… The first broadly available PD “modality” was truly continuous, as it spanned a 24/7 delivery cycle. Continuous PD became possible through the technique advances made Popovich and Moncrief, and became known as continuous ambulatory peritoneal dialysis (CAPD).
CAPD ruled the peritoneal firmament for 10-15 years while (in particular) the Baxter conglomerate worked out how to automate the process. In that period, PD was relatively easy: four (or occasionally five) bags a day, using manual exchanges, and delivered almost exclusively by a surgically inserted straight Tenckhoff catheter (TC). Then stuff began to change. Automation slowly arrived, and improved. Odd octopus and gantry-style machines evolved to deliver PD fluid. Different versions of the abdominal TC emerged, some spiral-shaped, some with disc separators, the Toronto Western catheter…all attempting to improve on Henry Tenckhoffs’ original design. Catheters that exited through the skin above the sternum appeared. Soon, automation brought the option of a night-time-only version to PD.
Suddenly, PD split into two primary options: manual 24/7 CAPD, and an overnight 10-12/24 automated PD (APD) choice. But then, sometimes overnight-only APD proved to be not quite enough, so, the option of a single manual exchange during the day in addition to overnight APD seemed to suit some patients better. An array of fluid options emerged. TCs began to be implanted at the bedside, or in radiology, and not the operating theatre. PD was moving, changing, morphing into a smorgasbord of micro-choice.
Now, while PD is still PD, the wide array of differing options can create problems for studies, or for patient educators, when trying to compare one “modality” with another or to assist patients in choosing what might suit their needs best. Is APD really the same as CAPD? Is APD with Physioneal® and/or Icodextrin® the same as CAPD with Dianeal®?
- Well, yes, they are, at least in principle. They all use the peritoneal cavity.
- But, no, they aren’t, as the chosen option(s) may significantly impact efficacy, and alter the complication and infection rates.
In my view, APD is a different beast than CAPD…and it is sufficiently different to be (at the least) a different sub-modality. Studies should evaluate it in its own right vs CAPD, rather than lumping both together in both studies and registries. Too often, “PD” is referenced as an undifferentiated, amorphous modality when, in truth, the nuances matter.
Next, Haemodialysis (HD): a Proper Minefield
First, there is facility-based 3x week HD. Easy, right? No, wrong! Three times weekly HD delivered at a facility is not a uniform modality: it varies, jurisdiction-to-jurisdiction, country-by-country, around the world. US-style in-facility HD (= 3 x 3.0-3.5 hour) is not the same as Japanese or ANZ-style in-facility HD (= 3 x 4.5-5.0 hour). Note that this supposedly “one” modality actually tries to equate 9-11.5 hours HD/week with 13.5-15 hours HD/week …as if they are the same.
Are they comparable? Probably not, especially when it comes to the incomparable UFR commonly required by each. Yet, they are all-too-often compared and contrasted as if they are the same. They both likely do cut the mustard for a minimum Kt/V urea, though the US model more likely just gets there, while the Japanese/ANZ model more likely well exceeds. But they are chalk and cheese when it comes to comparable (or rather incomparable) rates of volume removal; a distinction that likely matters enormously when it comes to cardiovascular outcomes. Yet, both are often compared as if they are the same. After all, they are both facility-based, and that is often what seems to be taken to matter—and not the quality of the treatment provided.
Home HD is even more complex. While short daily HD (SDHD) can, of course, be offered in-facility—incidentally, a factor that complicates the interpretation of global in-facility dialysis data—it is a common option used (especially in the US) for the delivery of home HD via the NxStage System One. But, while SDHD (at home) is clearly different to long nightly HD (at home), both are often conflated as “home HD.” But, what do each of these really mean?
- SDHD can be short-short (5-6/week x 2-2.5 hours/treatment) or mid-short (5-6/week x 3-ish hours/treatment), yet both are called SDHD. This is despite the math that tells me that that one is as few as 10 hours/week (5 x 2) while the other offers as much as 18 hours/week (6 x 3) of membrane contact time (MCT) each week. One is nearly double the MCT of the other, yet both are often confused under the single banner of SDHD. Are they the same? I don’t think so.
- Meanwhile, nocturnal HD can be clinic-delivered, or delivered at home. If clinic-sited, it is never more than 3 x week. From my reading of US practices, it is offered in variants from 3 x 5-6 (15-18 hours/week) to 3 x 8 (24 hours/week), a likely highly significant difference in MCT. Yet even more importantly, the greatest threat—the “Killer gap” first emphasized by Kjellstrand—remains. (NB: clinic NHD is not an offered option for nocturnal HD in ANZ.)
How can these “modalities” be compared to the Canadian or ANZ 6 x 8 hours/week models of nocturnal home HD? The latter models deliver up to 48 hours of MCT/week with no extended inter-dialytic break. These regimens, often referred to as extended hour and frequency, or “intensive” nocturnal home dialysis cannot fairly or truly be compared with an NHD model (facility-based) that offers less than half the number of hours/week and with a long break as well! Although all are commonly conflated as one overarching therapy (or modality)...NHD…indeed, they are far from the same.
The most common NHD variant in ANZ is the alternate night model (3.5 x 8 hours/week) that offers 28 hours/week without a long break. This is an option that just might be the best compromise of all, though I still struggle (just a little) to put that in writing! But, again, the alternate night home NHD option, along with 3 x week in-facility NHD, and the Pierratos intensive home NHD modality, are all called NHD, and are too often compared, often quite undifferentiated, as if they were like options, or one modality.
Then, if incremental dialysis starts were to catch on, or if haemodiafiltration (HDF) should take root in the US (see this post as well), the modality mayhem would increase even further.
But…There is More
None of this scratches the surface of the smorgasbord of differences in blood flow rates, the vexed issue of blood to dialysate flow ratios, the differing dialysate flow rates in differing HD systems, the variations in dialyser surface area and/or membrane type, the dialysate temperature as a key factor in less symptomatic dialysis, the differences in access and access capacity, and all-important variables like ultrafiltration volume and its relevance to sessional duration through its key intercept: ultrafiltration rate.
All of these matter. All change the dynamics of HD significantly. Yet, rarely are they well delineated or standardised in studies that blithely compare modality “x” with modality “y.” As no two runs are the same, as no two sets of settings are the same, and as no two patients are the same, studies that compare modality “x” with modality “y” will ever remain confounded from the start.
So, yes, it is getting messy—very messy indeed. Well-intentioned literature comparisons all-too-often use labels like facility-based, in-centre, PD, SDHD, or NHD, indeed, sometimes just “home dialysis” without even attempting to make a distinction between home PD (CAPD or APD) or home HD (in its many forms and options). Even where the distinctions are made, few attempt to segregate regimens by MCT, flow rates, and/or a likely estimate of the exchangeable volume-past-the-post.
How, then, can our patients negotiate this tangled web to wisely choose their optimum modality when the most important variable of all—lifestyle—enters the fray?
Importantly, too, I have read many patient-to-patient comments and seen much heart-felt advice at social media sites, made without doubt in all good faith and with the best intent, that extoll the virtues and traps in option choice and describe the personal outcomes ascribed to modality “x” or modality “y”, all shared with great conviction—when it seems clear that the options being debated are quite different. Sadly, though, it often seems that these differences go unrecognised. This often adds to, rather than lessens, the confusion as, in response to a plea for help from a new-to-dialysis questioner, long multi-commented “advice” rollicks back and forth…some commenting PD, others describing variants of HD…to the complete confusion of the original question and questioner.
In my view, only four things end up counting:
- The lifestyle aspirations of the patient (PD or HD).
- The weekly membrane contact time (HD): the time allowable for solute and volume removal, and the shorter the total, the worse.
- The inter-dialytic interval (HD): where the key comparator cut-off ought be the inter-dialytic gap … one that never exceeds 43 hours (based on a 5 hour Rx) vs. any option with an interval that does.
The site of delivery: whether facility (HD) or home (PD or HD).
- Track 5-1Alternative renal replacement therapies in end-stage renal disease
- Track 5-2Choosing a modality for chronic peritoneal dialysis
- Track 5-3Chronic intermittent high-volume hemodiafiltration
- Track 5-4Dialysis modality and patient outcome
- Track 5-5Home hemodialysis
- Track 5-6Organization and elements of a home hemodialysis program
- Track 5-7Short daily hemodialysis
- Track 5-8Short daily home hemodialysis: The low dialysate volume approach
- Track 5-9Technical aspects of hemodiafiltration
- Track 5-10Technical aspects of nocturnal hemodialysis
- Track 6-1Clinical manifestations and diagnosis of peritonitis in peritoneal dialysis
- Track 6-2Fungal peritonitis in continuous peritoneal dialysis
- Track 6-3Hepatitis B virus and dialysis patients
- Track 6-4Hepatitis C virus infection in patients on maintenance dialysis
- Track 6-5Human immunodeficiency virus and dialysis
- Track 6-6Immunizations in patients with end-stage renal disease
- Track 6-7Microbiology and therapy of peritonitis in continuous peritoneal dialysis
- Track 6-8Non-access-related infections in chronic dialysis patients
- Track 6-9Pathophysiology and prevention of peritonitis in peritoneal dialysis
- Track 6-10Tunneled, cuffed hemodialysis catheter-related bacteremia
- Track 7-1Acute Hemodialysis Prescription
- Track 7-2Dialysis-Related Factors That May Influence Recovery of Renal Function in Acute Kidney Injury (Acute Renal Failure)
- Track 7-3Renal Replacement Therapy (Dialysis) In Acute Kidney Injury in Adults: Indications, Timing, and Dialysis Dose
- Track 7-4Renal Replacement Therapy (Dialysis) In Acute Kidney Injury: Metabolic and Hemodynamic Considerations
- Track 7-5Use of Peritoneal Dialysis for the Treatment of Acute Kidney Injury in Adults
- Track 7-6Clinical manifestations and diagnosis of coronary heart disease in end-stage renal disease (dialysis)
- Track 7-7Measurement of solute clearance in continuous peritoneal dialysis: Kt/V and creatinine clearance
- Track 8-1Darbepoetin alfa for the management of anemia in chronic kidney disease
- Track 8-2Diagnosis of iron deficiency in chronic kidney disease
- Track 8-3Effects of anemia in chronic kidney disease
- Track 8-4Hypertension following erythropoietin (EPO) in chronic kidney disease
- Track 8-5Hyporesponse to erythropoiesis-stimulating agents (ESAs) in chronic kidney disease
- Track 8-6Pure red cell aplasia due to anti-erythropoietin antibodies
- Track 8-7Treatment of anemia in hemodialysis patients
- Track 8-8Treatment of anemia in nondialysis chronic kidney disease
- Track 8-9Treatment of anemia in peritoneal dialysis patients
- Track 8-10Treatment of iron deficiency in hemodialysis patients
- Track 8-11Treatment of iron deficiency in nondialysis chronic kidney disease (CKD) patients
- Track 8-12Treatment of iron deficiency in peritoneal dialysis patients
- Track 9-1Evaluation of sudden cardiac arrest and sudden cardiac death in dialysis patients
- Track 9-2Hypertension following erythropoietin (EPO) in chronic kidney disease
- Track 9-3Hypertension in dialysis patients
- Track 9-4Inflammation in renal insufficiency
- Track 9-5Myocardial dysfunction in end-stage renal disease
- Track 9-6Pericarditis in renal failure
- Track 9-7Risk factors and epidemiology of coronary heart disease in end-stage renal disease (dialysis)
- Track 9-8Secondary prevention of cardiovascular disease in end-stage renal disease (dialysis)
- Track 9-9Serum cardiac biomarkers in patients with renal failure
- Track 9-10Therapy of heart failure in hemodialysis patients
- Track 9-11Treatment and prevention of sudden cardiac arrest in dialysis patients
- Track 9-12Treatment of coronary heart disease in end-stage renal disease (dialysis)
- Track 9-13Valvular heart disease in patients with end-stage renal disease
- Track 10-1Acute complications during hemodialysis
- Track 10-2Dialysis disequilibrium syndrome
- Track 10-3Intradialytic hypotension in an otherwise stable patient
- Track 10-4Muscle cramps in dialysis patients
- Track 10-5Seizures in patients undergoing hemodialysis
- Track 11-1Anticoagulation for continuous renal replacement therapy
- Track 11-2Continuous arteriovenous hemodialysis: Technical considerations
- Track 11-3Continuous renal replacement therapy in acute kidney injury (acute renal failure)
- Track 11-4Continuous venovenous hemodiafiltration: Technical considerations
- Track 11-5Continuous venovenous hemodialysis: Technical considerations
- Track 11-6Drug removal during continuous renal replacement therapy
- Track 11-7Outcomes associated with nocturnal hemodialysis
- Track 11-8Sustained low efficiency or extended daily dialysis
- Track 11-9Central catheters for acute and chronic hemodialysis access
- Track 12-1Adynamic bone disease associated with chronic kidney disease
- Track 12-2Carbohydrate and insulin metabolism in chronic kidney disease
- Track 12-3Cortisol metabolism in chronic kidney disease
- Track 12-4Dialysis in diabetic nephropathy
- Track 12-5Growth hormone metabolism in chronic kidney disease
- Track 12-6Indications for parathyroidectomy in end-stage renal disease
- Track 12-7Management of hyperglycemia in patients with type 2 diabetes and pre-dialysis chronic kidney disease
- Track 12-8Management of secondary hyperparathyroidism and mineral metabolism abnormalities in adult
- Track 12-9Management of secondary hyperparathyroidism and mineral metabolism abnormalities in dialysis patients
- Track 12-10Overview of chronic kidney disease-mineral and bone disorder (CKD-MBD)
- Track 12-11Reproductive and sexual dysfunction in uremic women
- Track 12-12Sexual dysfunction in uremic men
- Track 12-13Thyroid function in chronic kidney disease
- Track 12-14Treatment of hyperphosphatemia in chronic kidney disease
- Track 13-1Drug removal during continuous renal replacement therapy
- Track 13-2Enhanced elimination of poisons
- Track 13-3General approach to drug poisoning in adults
- Track 13-4Isopropyl alcohol poisoning
- Track 13-5Meprobamate poisoning
- Track 13-6Methanol and ethylene glycol poisoning
- Track 13-7Paraquat poisoning
- Track 13-8Salicylate (aspirin) poisoning in adults
- Track 13-9Theophylline poisoning
- Track 13-10Valproic acid poisoning
- Track 14-1Assessment of nutritional status in hemodialysis patients
- Track 14-2Leptin and end-stage renal disease
- Track 14-3Nutritional status and protein intake in peritoneal dialysis patients
- Track 14-4Pathogenesis and treatment of malnutrition in maintenance hemodialysis patients
- Track 14-5Protein intake in maintenance hemodialysis patients
- Track 14-6The nephrologist as primary care clinician in patients with end-stage renal disease
- Track 15-1Medical management of the dialysis patient undergoing surgery
- Track 15-2Continuous renal replacement therapies: Overview
- Track 15-3Withdrawal from and withholding of dialysis
- Track 15-4Uremic toxins
- Track 15-5Uremic pruritus
- Track 15-6Uremic polyneuropathy
- Track 15-7Uremic mononeuropathy
- Track 15-8Unique aspects of gastrointestinal disease in dialysis patients
- Track 15-9Platelet dysfunction in uremia
- Track 15-10Palliative care: End-stage renal disease
- Track 15-11Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure
- Track 15-12Aluminum toxicity in chronic kidney disease
- Track 15-13Lower extremity peripheral artery disease in end-stage renal disease
- Track 15-14Indications for initiation of dialysis in chronic kidney disease
- Track 15-15Hemoperfusion
- Track 15-16Hemodialysis in the older adult
- Track 15-17Eye disorders associated with chronic kidney disease
- Track 15-18Ethical issues in the care of the patient with end-stage renal disease
- Track 15-19Dialysis-related amyloidosis
- Track 15-20Complications of hemodialysis in the older patient
- Track 15-21Cancer screening in patients with end-stage renal disease
- Track 15-22Calciphylaxis (calcific uremic arteriolopathy)
